Clinical background
DEFINITIONS
Occupational medicine is the branch of medicine concerned with the maintenance of health in the workplace, including prevention and treatment of diseases and injuries, with secondary objectives of maintaining and increasing productivity and social adjustment in the workplace. Such matter is regulated in the European Union by common basis described in the Council Directive 89/391/ECC. This piece of legislation includes:
- Assessment of working conditions (risk assessment)
- Provision of recommendations for improvement of working conditions
- Informing and advising the employees about work-related risks
- Early recognition and prevention of occupational diseases and work-related illnesses
- Improving the knowledge of exposure and risks
Occupational medical care not only aims to keep employees healthy, but also to improve health protection at the workplace. Occupational toxicology, a branch of occupational medicine, is the application of the principles and methodology of toxicology toward chemical and biologic hazards encountered at work. The objective of the occupational toxicologist is to prevent adverse health effects in workers that result from their work environment. [1][2] A great efforts focus on biomonitoring, defined as the analysis of biological material obtained from employees for the presence of hazardous substances and/or their metabolites. The goal of biomonitoring is to determine the internal exposure of workers in order to compare the analytical results with threshold values and suggest appropriate measures to reduce exposure. [3]
There are two kinds of biomonitoring: internal exposure monitoring and biological effect monitoring. “Internal exposure monitoring”determines the levels of hazardous substances while the “biological effect monitoring”studies the biological reactions at cellular level ( e.g. cytotoxicity, mutations) through the quantification of reaction products of mutagenic substances (e.g DNA or protein adducts). [4]
PARAMETERS OF BIOMONITORING
In clinical diagnosis, the most used biological materials are whole blood, plasma and urine as 24-h pooled urine samples. If these cannot be used, the analyses must be made on spontaneous urine samples and the results must be expressed in terms of creatinine levels, to take into account diuresis-induced variations. Moreover, a reliable biomonitoring process that is indicative of the exposure requires samples taken at specified times. Information on toxicokinetics may be found in the International Chemical Safety Cards (ICSCs). [5] Generally, the materials for analysis are collected at the end of a shift, if possible after 3 days at work.
Figure 1 and Figure 2 recap the required biological material and the times of sampling for biomonitoring parameters, included (Figure 1) or not included (Figure 2) in the Guidelines for Occupational Medical Examinations. [6]
 Figure 1. Required biological material and time of sampling for biomonitoring parameters included in the Guidelines for Occupational Medical Examinations.
Figure 1. Required biological material and time of sampling for biomonitoring parameters included in the Guidelines for Occupational Medical Examinations.
 Figure 2. Required biological material and time of sampling for biomonitoring parameters not included in the Guidelines for Occupational Medical Examinations.
Figure 2. Required biological material and time of sampling for biomonitoring parameters not included in the Guidelines for Occupational Medical Examinations.
Results are evaluated by comparison with occupational medical and environmental threshold values that can be found in the list of MAK and BAT values. [7]
The analytical laboratories certified for occupational toxicology must use up-to-date analytical methods, carry out regular internal and external quality control and support the occupational physician in the interpretation of analytical results. Is then required that the involved laboratory has appropriate expertise and apparatus and make use of reliable analytical methods.
The case study of acetone in gc-fid
Acetone is a chemical in the form of a colorless liquid with a distinct smell and taste, naturally found in the environment or produced by industries. Low levels of acetone are normally present in the body from the breakdown of fat and are used to make sugar. The smell of acetone can be detected in air at 100 to 140 ppm and in water at 20 ppm. Acetone evaporates readily into the air and mixes well with water. Most of the industrially produced acetone is used to make plastics, fibers and drugs, or to dissolve other substances. Acetone is also released from automobiles exhaust and from tobacco smoke, landfills, and certain kinds of burning waste materials. Consequently, people who work in paint, plastic, artificial fiber, and shoes factories along with professional painters and commercial and household cleaners,can be exposed to higher acetone levels than the general population. [8]
Acetone irritates noses, throats, lungs, and eyes. The irritation can be felt at levels of 100 ppm in the air. Workers exposed to acetone (from 2 minutes to 4 hours) at 12,000 ppm or higher also complain of headaches, lightheadedness, dizziness, unsteadiness, and confusion. Moreover, changes in the ovulation period were reported from women exposed continuously to 1,000 ppm of acetone for about 8 hours. Liquid acetone applied the skin leads to the development of skin irritation with cell damaging. However, the Department of Health and Human Services and the International Agency for Research on Cancer have not classified acetone as carcinogenic. [8]
Biomonitoring of acetone is usually made in urine. This measurement determines whether the worker have been exposed to acetone (the levels are higher than those normally seen) and the severity of exposition (through the comparison with MAK and BAT values).
EXPERIMENTAL CONDITIONS
The method for Acetone analysis in urine was developed using gas chromatography (Nexis GC-2030 Shimadzu) with a flame ionization detector (FID). Samples were diluted in double distilled water in a volumetric ratio 1:2 urine-water. Chromatographic separation was obtained on a Phenomenex ZB-WAXplus30 m 0.25 mm 0.25 μm with helium as carrier gas. Fig 3 recaps the optimized chromatographic conditions.
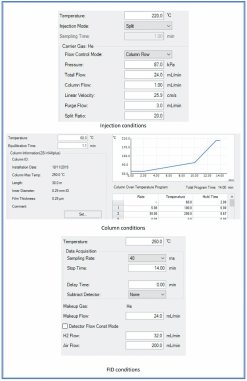 Fig. 3 Optimized chromatographic conditions for Acetone analysis in GC-FID.
Fig. 3 Optimized chromatographic conditions for Acetone analysis in GC-FID.
Figure 4 shows the retention time (Rt) and the obtained chromatogram of an exposed worker with Acetone concentration above the threshold of MAK and BAT values.
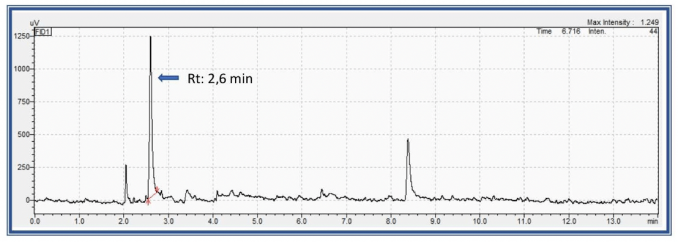 Fig. 4 GC-FID chromatogram of Acetone in urine.
Fig. 4 GC-FID chromatogram of Acetone in urine.
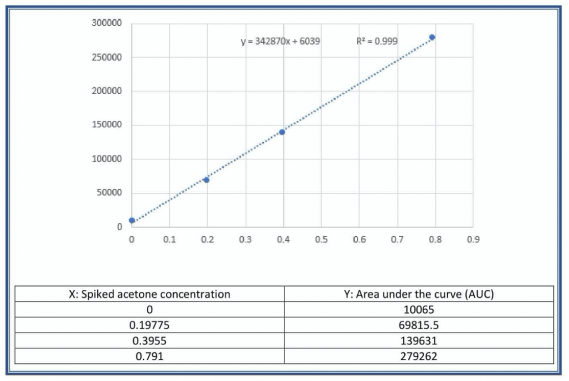 Fig. 5 Calibration curve obtained through Acetone standard addition in urine.
Fig. 5 Calibration curve obtained through Acetone standard addition in urine.
Table 1 lists the measured values of correlation coefficient, relative deviation, low limit of detection and low limit of quantification of the optimized method.
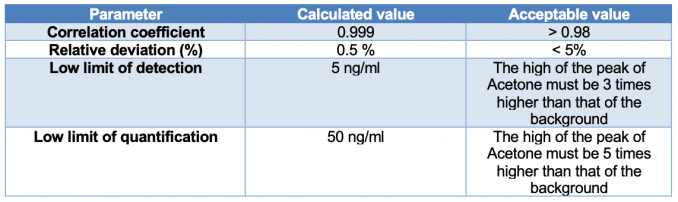 Table 1. Parameters of quality for the optimized method.
Table 1. Parameters of quality for the optimized method.
From these data, it is understandable that standard error and standard deviation are low. Consequently, the method has high repeatability and reproducibility. Moreover, the effectiveness of the method was quantified through recovery experiments (the fraction of the analyte determined after addition of a known amount of the analyte to a sample) and the study of the robustness against variations (like temperature, pH, moisture etc…) (Table 2).
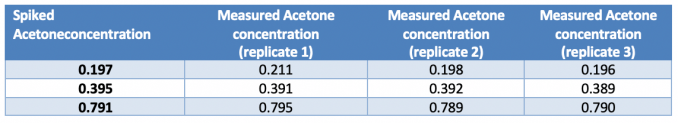 Table 2. Recovery experiments.
Table 2. Recovery experiments.
The procedures of external quality control along with the elevated number of clinical determinations carried out with this method confirmed the robustness of the assay.
In conclusion, the described analysis of Acetone in GC-FID can act as a guidance for the appropriate expertise and apparatus needed for the development of reliable analytical methods.
Author

Dr Raffaele Conte, PhD, FIScT, RSci is the director and responsible chemist of the toxicology laboratory of AMES polydiagnostic centre in Casalnuovo, Italy and a research associate of the Research Institute on Terrestrial Ecosystems of the Italian National Research Council (CNR) in Naples, Italy. Apart from his working activity on the detection of drugs and metabolites in biological matrices, his research interests are focused on the design of novel delivery platforms able to minimize degradation, prevent undesirable side effects, and increase bioavailability of drugs or genetic materials. He also follows different projects as an independent researcher and has authored several research papers. In 2018 he obtained a PhD in clinical and experimental medicine at the University of Naples “Federico II” with a project on the synthesis of polymeric fillers with anti-biofilm activity for dental composites. His email address is raffaele.conte86@tiscali.it
REFERENCES
- Groneberg DA, Fischer A. Occupational medicine and toxicology. J Occup Med Toxicol. 2006;1:1-. doi:10.1186/1745-6673-1-1.
- Carter JT. Occupational toxicology. Human toxicology. 1988;7(5):429-32. doi:10.1177/096032718800700508.
- Paustenbach D, Galbraith D. Biomonitoring and biomarkers: exposure assessment will never be the same. Environmental health perspectives. 2006;114(8):1143-9. doi:10.1289/ehp.8755.
- Tranfo G. The Growing Importance of the Human Biomonitoring of Exposure. International Journal of Environmental Research and Public Health. 2020;17(11):3934.
- WHO. International Chemical Safety Cards (ICSCs). 2020.
- DGUV. Guidelines for Occupational Medical Examinations. 2020. https://www.dguv.de/en/prevention/topics-a-z/med_prophylaxis/guidelines/index.jsp.
- List of MAK and BAT Values 2019. List of MAK and BAT Values 2019. 2020. p. COVER-XXVIII.
- (ATSDR) AfTSaDR. Toxicological profile for acetone. Department of Health and Human Services, Public Health Service. 1994.
